- Home
- What we do
- Contract manufacturing
- Injectables contract manufactu...
High-quality sterile injectable manufacturing
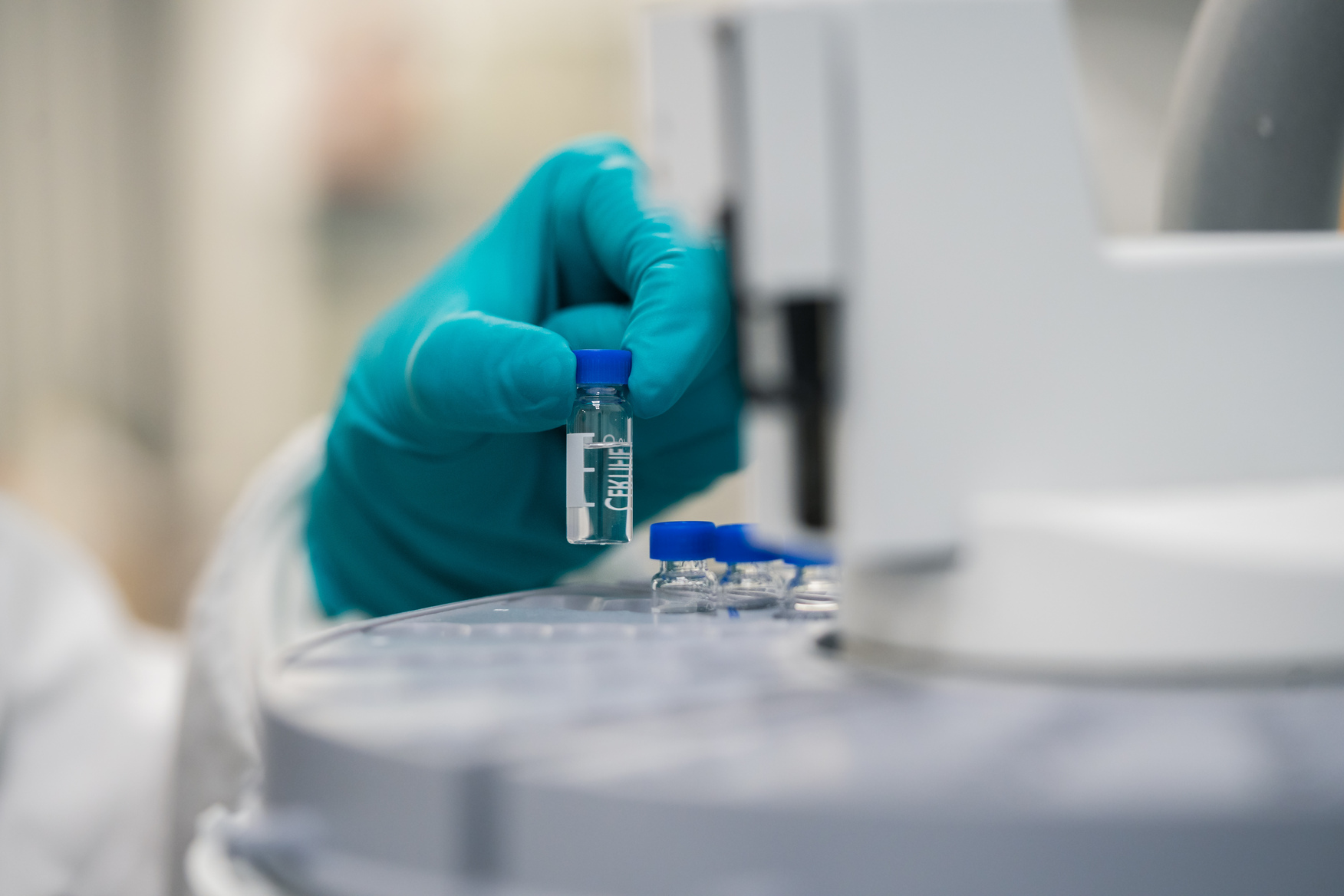


When you need a trusted partner for your sterile injectable drug products, chose Hikma. We guarantee high-quality, consistency and reliability at every stage. Scale-up processes from R&D level to industrial size. From pilot batches to commercial production with comprehensive fill and finish.
We produce 500 million units a year. And we are expanding our capacity in the EU and US. We offer a wide range of sterile injectable services.
Technology:
IV Bags
Prefilled syringes
Liquid vials
Lyophilised vials
Ampoules
Products:
General formulation liquid solutions
General formulation lyophilisation
Liquid lyophilised biologics
Emulsions
Suspensions
Liquid and lyophilised cytotoxic and high-containment
Cephalosporins
Controlled substances
5 state-of-the-art operational sites:
Bedford, US
Cherry Hill, US
Goslar, Germany
Sintra, Portugal
Pavia, Italy
Full compliance with quality and safety standards.
A strong regulatory track record.
US FDA, EMA, Japan FDA, Jordan FDA, Korea, GCC and ANVISA approved.
Over ten new filings and approvals in EU and US markets every year.
For high-quality, global reach, local capacity and flexible manufacturing, choose Hikma.
Fill-finish capabilities for vials, prefilled syringes, infusion bags, and ampoules across all formulation types. 500+ million unit annual capacity in EU and US








Our operations
It is the global hub for the Injectables business. It includes:
• Three segregated plants for the manufacturing of general formulation products, high containment products and cephalosporins antibiotics
• Our capabilities cover different forms and technologies, including vials and
ampoules, prefilled syringes, IV bags, emulsions/suspensions and oils
• The site services, the US, EU, UK and MENA markets.
Our liquid and lyophilisation cytotoxic hub for the US, Europe and MENA.
Our lyophilisation hub for Europe and MENA.
It produces a variety of injectables products in various forms, including prefilled syringes.
Our newest site for septic bags filling and lyophilisation for the US.
High-quality prefilled manufacturing operations in the US
Get in touch
Contact us to discuss your injectable CMO needs and book a tour in our facilities.
