Hikma launches Sodium Acetate Injection, USP, in the US
London, 11 July 2024 – Hikma Pharmaceuticals PLC (Hikma), the multinational pharmaceutical company, has launched Sodium Acetate Injection, USP, in 40 mEq per 20 mL vials in the US. The product was approved with a Competitive Generic Therapy (CGT) designation from the US Food and Drug Administration and is therefore eligible for 180 days of CGT exclusivity. To help address the shortage of this critical product, which is currently impacting hospitals and patients across the US, Hikma will be prioritising the supply of the 20 mL vials. Hikma will also launch Sodium Acetate Injection, USP in 100 mEq per 50 mL and 200 mEq per 100 mL vials at a future date.
Sodium Acetate Injection, USP is indicated as a source of sodium for addition to large volume intravenous fluids to prevent or correct hyponatremia in patients with restricted or no oral intake. It is also used as an additive for preparing specific intravenous fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions.
According to IQVIA, US sales of Sodium Acetate Injection, USP, 40 mEq per 20 mL,100 mEq per 50 mL and 200 mEq per 100 mL were approximately $26 million in the 12 months ending May 2024.
Hikma is a top three supplier of generic injectable medicines by volume in the US1 , with a growing portfolio of more than 160 products. We are continuously expanding our portfolio of essential medicines and introducing new dosage forms that enhance patient care.
1Source: IQVIA MAT May 2024, generic injectable volumes by eaches, excluding branded generics and Becton Dickinson
This product has been approved for marketing in the United States by the US FDA. This product approval does not confer the right on Hikma, or any other party, to market this product outside the United States.
Important Safety Information for Sodium Acetate Injection, USP, 40 mEq per 20 mL:
Please see package insert for referenced section/section numbering, where appropriate.
CONTRAINDICATIONS
Sodium acetate injection is contraindicated in patients with hypernatremia or fluid retention.
WARNINGS & PRECAUTIONS
- Sodium acetate injection must be diluted before use.
- To avoid sodium overload and water retention, infuse sodium-containing solutions slowly.
- Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
- In patients with diminished renal function, administration of solutions containing sodium ions may result in sodium retention.
- Solutions containing acetate ions should be used with great care in patients with metabolic or respiratory alkalosis. Acetate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion, such as severe hepatic insufficiency.
- The intravenous administration of this solution (after appropriate dilution) can cause fluid and/or solute overloading resulting in dilution of other serum electrolyte concentrations, overhydration, congested states or pulmonary edema. Excessive administration of potassium free solutions may result in significant hypokalemia.
- This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
- Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
- Do not administer unless solution is clear and seal is intact. Discard unused portion.
- Sodium replacement therapy should be guided primarily by the serum sodium level.
- Caution should be exercised in administering sodium-containing solutions to patients with severe renal function impairment, cirrhosis, cardiac failure, or other edematous or sodium retaining states, as well as in patients with oliguria or anuria.
- Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
- Solutions containing acetate ions should be used with caution as excess administration may result in metabolic alkalosis.
ADVERSE REACTIONS
Sodium overload can occur with intravenous infusion of excessive amounts of sodium-containing solutions.
USE IN SPECIFIC POPULATIONS
Pregnancy
Animal reproduction studies have not been conducted with sodium acetate injection. It is also not known whether sodium acetate injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium acetate injection should be given to a pregnant woman only if clearly needed.
Pediatric Use
Safety and effectiveness have been established in the age groups infant to adolescent.
Geriatric Use
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Sodium ions are known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
DOSAGE AND ADMINISTRATION
Sodium acetate injection (2 mEq/mL) is administered intravenously only after dilution in a larger volume of fluid. The dose and rate of administration are dependent upon the individual needs of the patient. Serum sodium should be monitored as a guide to dosage. Using aseptic technique, transfer the desired amount to other intravenous fluids to provide the appropriate number of milliequivalents (mEq) of sodium acetate.
Sodium acetate injection in the Pharmacy Bulk Package is designed for use with manual, gravity flow operations and automated compounding devices for preparing intravenous nutritional admixtures. Admixtures must be stored under refrigeration and used within 24 hours after compounding.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Directions for Dispensing From Pharmacy Bulk Package
The Pharmacy Bulk Package is for use in the Pharmacy Admixtures Service only. For hanger application, peel off the paper liner from both ends of the tape hanger to expose ¾ inch long adhesive portions. Adhere each end to the label on the bottle. The vials should be suspended as a unit in the laminar flow hood.
A single entry through the vial closure should be made with a sterile dispensing set or transfer device. Transfer individual doses to appropriate intravenous infusion solutions. Use of a syringe with needle is not recommended as it may cause leakage and multiple entries will increase the potential of microbial and particulate contamination.
The above process should be carried out under a laminar flow hood using aseptic technique. Discard any unused portion within 4 hours after initial closure entry.
OVERDOSAGE
In the event of overdosage, discontinue infusion containing sodium acetate immediately and institute corrective therapy as indicated to reduce elevated serum sodium levels, and restore acid-base balance if necessary.
INDICATIONS AND USAGE
Sodium acetate injection is indicated as a source of sodium for addition to large volume intravenous fluids to prevent or correct hyponatremia in patients with restricted or no oral intake. It is also useful as an additive for preparing specific intravenous fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions.
HOW SUPPLIED/STORAGE AND HANDLING
Sodium acetate injection USP, 40 mEq (2 mEq/mL) is supplied as follows:

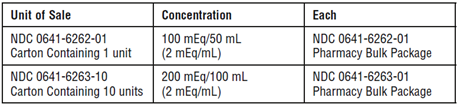
Sodium acetate injection USP, (2 mEq/mL) is supplied in Pharmacy Bulk Packages as follows:
Store at 20 to 25ºC (68 to 77ºF). [See USP Controlled Room Temperature.]
ENDING INFORMATION
For additional information, please refer to the Package Insert for full prescribing information, available on www.hikma.com.
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689 or FDA at 1-800 FDA-1088 or www.fda.gov/medwatch.
Distributed by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922 USA
Document Identification Number: HK-2558-v1
